RADIOACTIVE DECAY:
Ever heard of Plutonium? It's the stuff we use in our nuclear things -- weapons, submarines, etc. Plutonium-239 has a half-life of 24,110 years.
"Half-life means that, if you have 100 pounds of Plutonium-239...
In 24,110 years, you'd still have 50 pounds left...
In another 24,110 years, you'd still have 25 pounds left.
This stuff just won't go away! This is why it is such a big concern when a nuclear submarine sinks... Eventually, the salt water will eat through the steel and release the Plutonium (which, as you know, is quite lethal.) They usually talk about either trying to raise the sub or encase it in concrete where it rests. The last figure I heard was that there are currently eight nuclear subs on our ocean floors. Now that I've completely depressed you... back to the math!
Hey, did you know that YOU are radioactive? You've got this stuff in you called Carbon-14... It comes from cosmic rays that rain down on the earth (and us) from outer space. (By the way, you are mostly Carbon-12, which is not radioactive. That's why we are called "Carbon-based life forms." Man, I've really watched too much Star Trek.)
Scientists use Carbon-14 to make a guess at how old some things are -- things that used to be alive like people, animals, wood and natural cloths. It doesn't work for sea creatures and other things that are under water. Think about it... Cosmic rays can't get through the water.
Anyway, they make an estimate of how much Carbon-14 would have been in the thing when it died... Then they measure how much is left in the specimen when they find it. This is where the half-life comes in... the half-life of Carbon-14 is about 5730 years.
Here's one of the formulas they use:
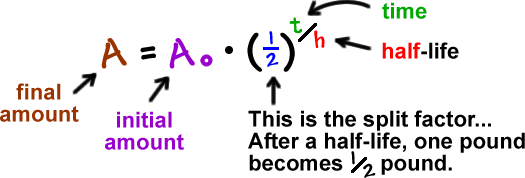
If we mess with this a bit, we can make it simpler:
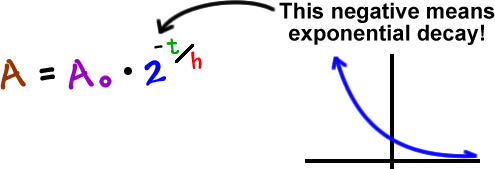
You can use either of these formulas. I'm going to use the second one since it's easier and it's used more often.