Let's walk through a problem without the formula first just to make sure that we get the concept of half-life:
In 2000, you buried 15 kg of Carbon-14 in your backyard. Someone digs it up in the year 13,460. How much Carbon-14 did they find?
OK, That's 11,460 years (which is two half-lives...)
After 5730 years, there'd be 7.5 kg.
After 5730 more years, there'd be 3.75 kg.
Now, let's do one with the formula:
You discovered a new radioactive isotope and named it boogonium (don't ask). It's half life is 1.23 years. If you start with a sample of 45 grams, how much will be left in 6.7 years?

|
Plug this stuff in!
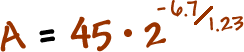
Grab a calculator!
![]()
YOUR TURN:
An alien radioactive isotope has a half-life of 238 years. If you start with a sample of 8 kg, how much will be left in 100 years?